Elusive protein protects malaria parasite from heme
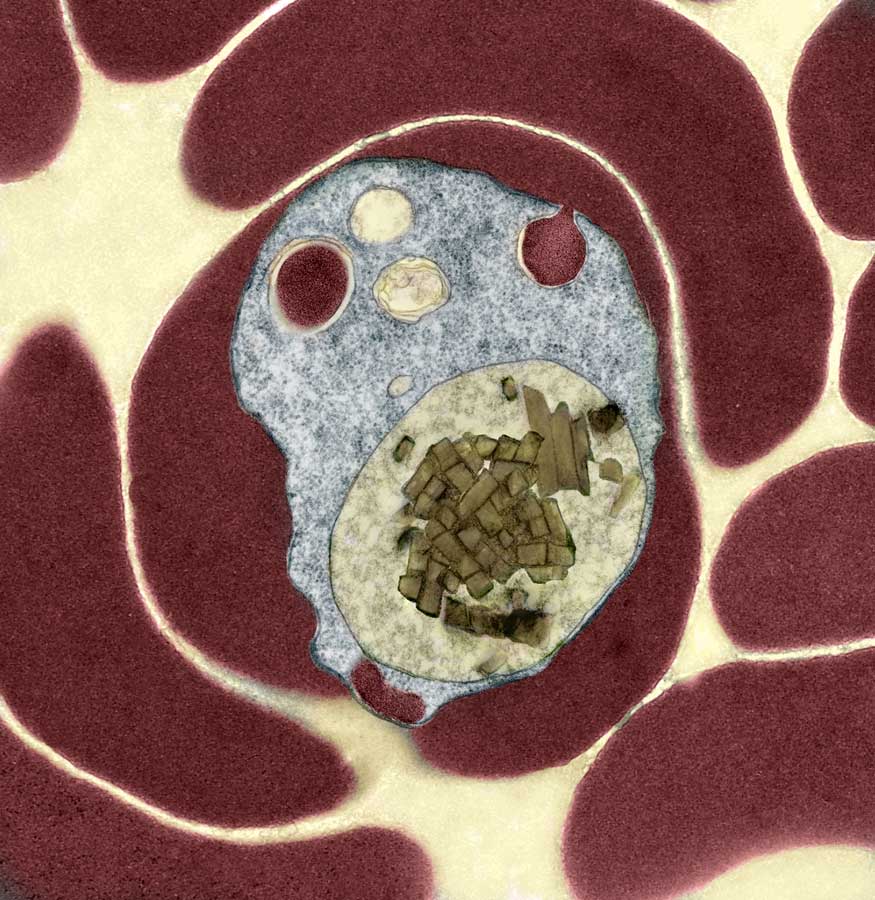
Researchers at the Virginia Bioinformatics Institute at Virginia Tech have identified Heme Detoxification Protein, a unique protein encoded in the malaria genome that represents a potential target for developing new malaria drugs.
The team, which includes researchers at Washington University School of Medicine, the United States National Institutes of Health, the United States Food and Drug Administration as well as other researchers at Virginia Tech, has characterized heme and demonstrated that it plays a major role in protecting Plasmodium as the pathogen pursues infection of its host. The findings were published April 28, 2008, in the open-access journal PLoS Pathogens (Jani D, Nagarkatti R, Beatty W, Angel R, et al. (2008) HDP-A Novel Heme Detoxification Protein from the Malaria Parasite. PLoS Pathogens 4(4): e1000053. doi:10.1371/journal.ppat.1000053).
Worldwide, the annual death toll of malaria exceeds 1 million, and children under the age of five are its major victims. The Plasmodium parasite that causes malaria in humans is transmitted through the bites of infected mosquitoes. Once inside the human body, the parasite initially develops in the liver and subsequently, upon release, infects red blood cells.
After infecting host red blood cells, a rapid growth ensues, supported by the parasite’s consumption of hemoglobin, the oxygen-transporting protein that constitutes a massive 90 percent of the total protein present inside each red blood cell. Destruction on this scale releases large quantities of heme, the prosthetic group responsible for oxygen transport in hemoglobin. Free heme is extremely damaging and to protect itself from this toxic onslaught, the parasite utilizes a novel mechanism where it rapidly converts heme into a crystalline material known as hemozoin.
Dharmendar Rathore, assistant professor at the institute, remarked: “We discovered [heme] as part of a functional genomics initiative that is focused on the identification of malaria proteins involved in disease pathology. A combination of cellular and biochemical approaches allowed us to rigorously characterize it. It appears that it has a number of striking features that make it a promising candidate as a drug target. Heme is not only capable of rapidly converting heme into its non-toxic counterpart hemozoin, but it is highly conserved in all the species of the parasite and also appears to be critical for its survival.”
He added: “The beauty of this discovery is that, while [heme] has robust interactions with heme, it lacks homology to any of the known heme-binding proteins and has therefore eluded detection during previous attempts by several groups to identify parasite factors responsible for hemozoin formation.”
The conversion of heme into hemozoin is regarded as one of the weakest links in the lifecycle of the Plasmodium parasite. For example, chloroquine, the most widely used malaria drug, works by interacting with heme and preventing its detoxification into hemozoin. However, drugs are not yet available that target any of the parasite-specific molecules involved in this process.
Rana Nagarkatti, research scientist at the institute, commented: “The identification of new drug targets is an essential step in the development of next-generation drugs for treating malaria. Drugs that specifically interact with heme and inhibit its detoxification activities could potentially have drastic effects on the viability of the malaria parasite.” Dewal Jani, a member of the institute’s research team, remarked: “The identification of heme fills an important gap in our understanding of the mechanism of hemozoin production in the malaria parasite. We have also established the route by which the Heme is transported out of Plasmodium and into the red blood cell before it subsequently returns to the parasite food vacuole where hemozoin is synthesized. This gives us an interesting insight into the inner workings of the parasite.”
Rathore concluded: “New drugs are urgently needed to address the huge public health burden posed by malaria across the globe. We have recently undertaken a high-throughput screening of chemical libraries to identify compounds that can inhibit the activity of [heme]. Several lead compounds were identified and have been characterized in our laboratory at the institute and subsequently validated at the Swiss Tropical Institute in Basel, Switzerland. We see considerable potential in developing these lead compounds into new drugs that can act by blocking the function of [heme] in the parasite.”
Otto Folkerts, associate director of technology development at the Virginia Bioinformatics Institute, added: “Virginia Tech Intellectual Properties Inc. has filed patents that cover the intellectual property behind this discovery. We are actively seeking partners who are interested in licensing this intellectual property or jointly pursuing the development of potential malaria drug candidates that may arise from this work.”
The work was supported by Virginia Tech-Johns Hopkins University infectious diseases research and ASPIRES (A Support Program for Innovative Research Strategies)program grants. The views expressed do not represent the official position of the U.S. Food and Drug Administration.



.jpg.transform/m-medium/image.jpg)
