Virginia Tech Carilion Research Institute scientist gets $2 million grant to study childhood virus

Sarah McDonald, an assistant professor at the Virginia Tech Carilion Research Institute, recently received a $2 million, five-year grant from the National Institutes of Health to study how a common — and, in the developing world, sometimes deadly — childhood virus builds itself anew.
Rotavirus infects nearly all children worldwide within the first five years of life. Despite decades of research, little is known about how the virus propagates. McDonald plans to change that.
“We’re seeking to close critical gaps in knowledge about how rotavirus replicates its genome in tandem with the early stages of virion particle assembly,” McDonald said. “That knowledge could foster the development of next-generation vaccines.”
Rotavirus causes severe diarrhea and extreme dehydration in children. Treatment is simple rehydration therapy, if the child has access to modern medical care.
In parts of the developing world, rotavirus kills as many as half a million children each year. By peering into the hidden production of rotaviruses, McDonald may learn more about how the virus works, which could lead to better vaccines.
“Rotaviruses replicate exquisitely,” said McDonald. “They simultaneously copy their genetic material as they assemble it into new virion particles.”
The rotavirus particle has a genome of 11 distinct segments of double-stranded RNA. The genome provides the virus the instructions to replicate, and proteins read the blueprint and begin a complex construction process.
Each single-stranded RNA template is first built into a "pre-core" intermediate structure. The 11 pre-core intermediate structures then come together to form a single core particle as 11 single-stranded RNA templates are simultaneously copied into double-stranded RNA segments.
“Remarkably, rotavirus performs this concerted replicase-assembly task within fortress-like structures, which the virus builds in the host cell cytoplasm,” said McDonald, also an assistant professor of biomedical sciences and pathobiology at the Virginia–Maryland Regional College of Veterinary Medicine. “Because of the natural properties of these structures, it has been difficult to see what’s going on inside them using traditional imaging approaches.”
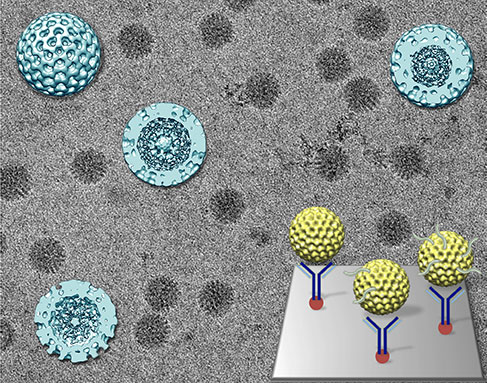
McDonald will use biochemical methods to break into the fortress and then look at the intermediate structures using immunoaffinity capture electron microscopy technology developed by a team of researchers led by Deborah Kelly, also an assistant professor at the Virginia Tech Carilion Research Institute.
This technology uses a tiny chip coated with specific antibodies to bind the virus without hindering function. The researchers image the sample with an electron microscope, compile the data, and reconstruct the structures as 3-D models.
“Our preliminary data revealed two novel structures,” McDonald said. “There’s a pebble-shaped complex that we suspect is the pre-core replication intermediate, and a particle-shaped complex that looks like the core replication intermediate.”
The first step of McDonald’s research will be to capture images of these structures in greater detail to create the first-ever architectural reconstructions of rotavirus replication intermediates.
“There’s the potential to apply the techniques we’re developing to other viruses, to elucidate their replication and assembly processes,” Kelly said.
The next step in the research will be to use other biochemical techniques to separate out interactions among key replication intermediates.
McDonald will focus on dissecting the interactions among three viral proteins: VP1, the enzyme that replicates the genome; VP2, the core shell protein that forms the innermost capsid layer of the virion; and NSP2, a doughnut-shaped protein that gives rise to the viroplasm formation — the secret fortress.
“Our hypothesis is that NSP2 binds to both VP1 and VP2 so it can regulate their interactions to prevent aberrant replication and premature core assembly,” McDonald said.
McDonald and her research team will determine exactly how VP1, VP2, and NSP2 interact before moving on to the third step in their research — determining how to disrupt these interactions to prevent rotavirus genome replication and assembly.
“We hope our research can go full circle,” McDonald said. “Understanding of form should lead to a better understanding of function. We believe this research could inspire the design of new, broadly effective vaccines for a range of viruses.”
Michael Friedlander, executive director of the Virginia Tech Carilion Research Institute, noted that the prestige of the award mechanism — the classic R01 research project grant — reaffirms the importance of McDonald’s work and her ability to achieve her goals.
“This grant, based on outstanding evaluations by the country’s leaders in molecular virology, attests to both Dr. McDonald’s scientific achievements and the importance and innovation of her proposed research," Friedlander said. "In this very competitive funding climate, the grant speaks directly to the high quality of her work and ideas. We’re fortunate to have her as part of both the research institute and the strong and growing community of virologists at Virginia Tech.”
Written by Ashley WennersHerron