Neuroscientist investigates how brain repairs itself after a stroke

A neuroscientist at the Virginia-Maryland Regional College of Veterinary Medicine at Virginia Tech says she hopes that a better understanding of how the brain restores blood flow to damaged tissue following a stroke will offer new treatment clues for a leading cause of death in the United States.
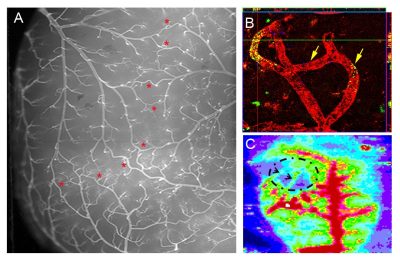
Michelle Theus, an assistant professor of molecular and cellular neurobiology in the Department of Biomedical Sciences and Pathobiology, is investigating how the brain develops “collateral” blood vessels which re-route blood flow after a vessel becomes blocked.
When the brain suffers from a blockage or clot, a network of replacement vessels, known as collaterals, can restore oxygen and nutrients to damaged tissue. The extent of the brain’s collateral network varies from individual to individual and has a significant impact on the brain’s ability to recover from stroke.
“It is widely known, clinically, that patients with an extensive collateral network have greater restoration of blood flow and are better protected from tissue damage following an embolic stroke,” said Theus, who explained that an embolic stroke involves a blood clot forming somewhere in the body and traveling through the bloodstream to the brain.
She is searching for answers about the brain’s natural network of collateral blood vessels, as well as its ability to remodel them after stroke and traumatic brain injury — two areas of study that remain largely a mystery. Last fall, Theus received a three-year, $483,000 grant from the National Institutes of Health to begin this research.
Theus hypothesizes that a family of Eph receptors— molecules that guide the development and growth of axons, or nerve fibers, in the brain — operate as “negative regulators” in the formation of collateral vessels. In other words, these receptors may make it difficult for the brain to initially form, as well as repair, collaterals and, in effect, limit the ability of the brain to mend itself after a stroke.
“If that’s the case, then therapeutic delivery of a drug that blocks this pathway may promote remodeling of the collateral network, improve blood flow, and limit tissue damage following stroke,” Theus said.
Although the study will provide new insights into the formation of collateral vessels and address the potential for developing a new technique for promoting their growth, Theus emphasized that research is in the preliminary stages.
“Our long-term goal is to identify a way to enhance with a blocker the collateral network of an individual at risk for stroke and to generate effective, safe, and feasible drug targets that improve collateralization during chronic repair and translate them into clinical applications,” she said.
Recent figures from the U.S. Centers for Disease Control and Prevention report that stroke claims almost 130,000 lives and costs the country an estimated $36.5 billion in health care costs and lost productivity each year.
According to Theus, her research has a much greater potential to impact human medicine than veterinary medicine. Unlike humans or even cats, dogs rarely experience symptoms of embolic stroke, likely due to the high number of collateral vessels.
Theus’ work is part of the growing One Health initiative that is uniting human and animal health. The One Health approach is dedicated to improving the lives of all species — human and animal — through the integration of human medicine, veterinary medicine, and environmental science. Efforts in the development and evaluation of new diagnostic methods, medicines, and vaccines can ultimately prevent and control of diseases across species.
She earned a bachelor’s degree in clinical laboratory sciences at the Ohio University and a doctorate in neuropathology and laboratory medicine at the Medical University of South Carolina.