Q&A: Virginia Tech researchers discover new, more effective candidates for treatment of syphilis
New candidates for the treatment of syphilis have been validated in a laboratory setting with several being more effective than what is currently used to treat the infection.
Brandon Jutras and Kathryn Hayes found new candidates for the treatment of syphilis with several being more effective than the current treatment options. Photo by Max Esterhuizen for Virginia Tech.

Since 2000, sexually transmitted infection rates have been on the rise. Syphilis, a disease that was nearly eradicated in the United States at that time, now affects more than 18 million people worldwide each year with few options for effective treatment.
One challenge that has plagued syphilis researchers for decades was the inability to culture and study the disease-causing agent in a laboratory setting.
“The incredible efforts of our colleagues and collaborators produced a faithful system to propagate the disease-causing agent in vitro, or in a laboratory setting. Being able to culture the bacterium opens new doors in terms of understanding it in terms of how it causes disease, ways we can prevent infection, and in efforts in which we may be able to intervene,” said Brandon Jutras, the principal investigator of the project, an assistant professor of biochemistry in the College of Agriculture and Life Sciences, and affiliated faculty of the Fralin Life Sciences Institute and the Center for Emerging, Zoonotic, and Arthropod-borne Pathogens.
Virginia Tech researchers set out to determine if there were possible treatment options that could serve as an alternative for the millions of people impacted by the disease each year.
What the College of Agriculture and Life Sciences researchers discovered exceeded all expectations. Not only did they find another treatment option to benzathine penicillin G, but they found two antimicrobial agents that were more effective in treating the disease-causing agent Treponema pallidum in a laboratory setting.
The research was published today in “Antimicrobial and Resistance,” a newcomer to the Nature Portfolio Journals, and was funded by the National Institutes of Health and the U.S. Department of Agriculture with additional internal funding from the Center of Emerging, Zoonotic, and Arthropod-borne Diseases.
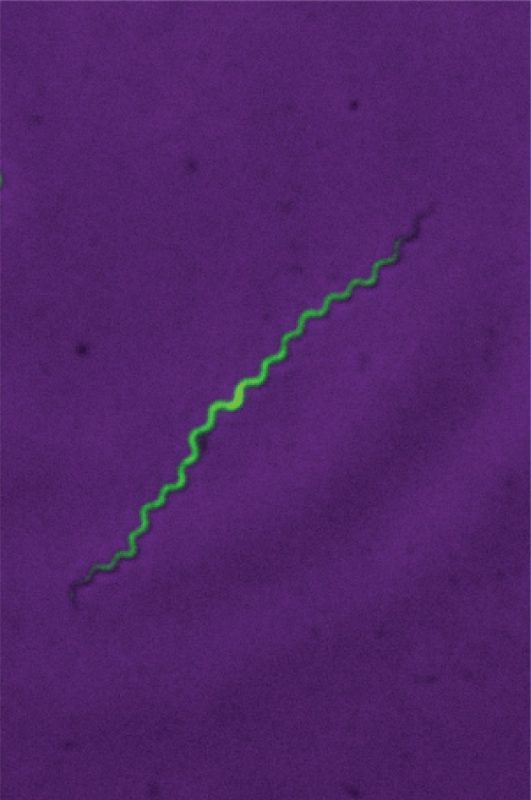
A fluorescent image of Treponema pallidum in a laboratory setting.
These drugs are already approved by the Food and Drug Administration, so they are safe for humans, which could accelerate the rollout.
“It’s a disease for which we have very few therapeutic options,” said Kathryn Hayes, the lead author and Ph.D. candidate in Virginia Tech’s Translational Biology, Medicine, and Health program. “We were able to do the first large-scale drug screen for syphilis treatment alternatives, efforts that would not have been possible without an in vitro culture system.”
Now, hear directly from the researchers on their project:
What inspired you to carry out this research?
Hayes: I have a huge passion for sexual health and a nerdy interest in infectious diseases. This research allowed me to combine these two interests as syphilis is a disease that has been around for centuries but little is known about it due to the difficulty of growing the bacteria in a lab setting. Having more data and research on the disease is a pressing clinical need.
What spurred your interest in sexual health?
Hayes: My own queer identity and how disproportionally impacted the queer community is by sexually transmitted diseases motivated me. The other is the stigma around sexually transmitted infections [STIs]. People will talk about how they have the flu, or even COVID-19, but no one will say they have syphilis. Education, or lack thereof, around sexual health, particularly STIs, has impacted how people talk about it and has continued to reinforce the importance of this research.
The research team of Kathryn Hayes and Brandon Jutras found two antimicrobial agents that were more effective in treating the syphilis disease-causing agent Treponema pallidum in a laboratory setting. Photo by Max Esterhuizen for Virginia Tech.

A year and 100 days: Can you describe the process you used to culture syphilis?
Hayes: The reason it is so hard is that the bacteria require very strict microaerophilic conditions – a low oxygen environment – which, in this case, means exactly 1.5 percent oxygen. We have an incubator that uses nitrogen to force out the excess oxygen so it keeps that exact percentage. The day before I culture, I take a supportive mammalian cell line and put it onto traditional plates, because they still need a co-culture to support growth. I have to make fresh media for the culture myself.
We have a few components that I make quarterly, and then every week I have to combine 12 ingredients to make the media, which then have to equilibrate overnight in our special incubator.
On average it was a two-hour prep the day before at minimum and then anywhere from like three to seven hours the day of depending on the number of bacteria I'm working with in that culture. As of last week, it has been a year and 100 days of culturing.
How did you conduct the drug screening?
Jutras: We started with nearly 100 antibiotics of a particular class, and two tetracyclines, which are in a different class of antibiotic, used as a cross-comparison. We incubated the bacteria with the antibiotics at an extraordinarily low concentration (five nanomolar) to get an initial reading of how effective they were at preventing growth.
From there, we took our top 25 percent of compounds and retested them to ensure that our analytical methods were accurate.
The top 10 percent were further investigated using sophisticated microscopy techniques in conjunction with antibiotic treatment. In essence, we could watch these compounds in action.
In addition, we determined their minimum inhibitory concentrations, and that's where we further confirmed that new candidates Azlocillin and Mezlocillin were more effective than our current standard of care, in vitro.
This research could have major implications. What’s next in the process for you?
Jutras: This project was a big risk. Addie could spend all this time and perform all this incredible research just to have discovered that nothing worked better than benzathine penicillin G. The surprising thing is that she found multiple options that work better.
Hayes: I want to look at modeling how these antibiotics are affecting bacteria. So, looking at protein drug interaction and how those interactions affect overall drug efficacy. I think that's a very interesting mechanistic step because once you understand what's happening to the bacteria on a molecular level, you can synthesize compounds that are very similar, but slightly different, to create more effective treatments.




