Rare disease research gives families hope, ‘vital’ to advancing medicine
Rare diseases afflict 300 million people worldwide. Fralin Biomedical Research Institute at VTC researchers are bridging a gap in scientific knowledge by studying some of the diseases that together add up to a formidable public health challenge. Feb. 28 marks the 14th international Rare Disease Day.
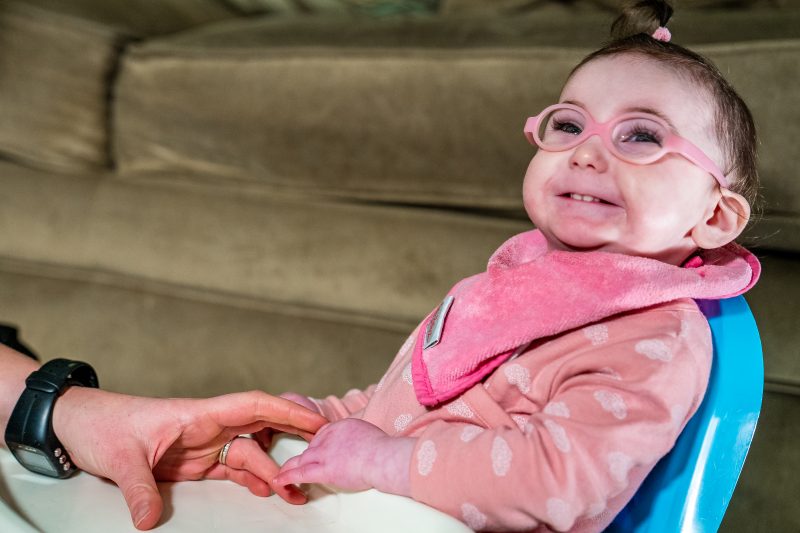
Six weeks after Evie was born near Dublin, Ireland, her doctors noticed she wasn’t hitting developmental milestones. Months later she was diagnosed with a currently untreatable genetic disorder – a rare mutation in the CASK gene. The gene provides instructions for making an important protein in brain cells that regulates expression of other genes involved in healthy brain development.
While her condition is extremely uncommon – reported in fewer than 60 females worldwide – it is one of 8,000 known rare diseases that collectively affect 10% of Americans.
Feb. 28 marks the 14th annual Rare Disease Day, an international effort to draw attention to the public health impacts of these diseases.
“Studying rare diseases not only helps patients living with these conditions, but it also teaches us about more common medical conditions,” said Marshall Summar, director of the Rare Disease Institute and the Margaret O’Malley Chair of Genetic Medicine at Children’s National Hospital, a clinical partner of Virginia Tech.
Summar is well-known for his pioneering work in researching and treating rare diseases in children. By studying rare diseases, such as urea cycle disorder, he’s advanced medical treatments for infants with more common conditions, including respiratory and heart disease.
Roughly 8% of rare diseases have therapies approved by the Food and Drug Administration, according to Summar.
“Just because you don’t have a drug, doesn’t mean you can’t care for a patient,” Summar said. “Progress is being made to discover new treatments, but there’s still so much ground to cover.”
Scientists at the Fralin Biomedical Research Institute at VTC are investigating rare diseases to identify new treatments while learning about key biological processes underlying brain development, cardiovascular disease, cancer, and neurorehabilitation.
Evie’s story – CASK disorder
“We were told that Evie would basically never do anything – she’d never be mobile, she’d never walk, she’d never talk,” said her father, Keith Purdy.
After receiving a grim prognosis, Evie’s parents took to the internet to find answers. They found a paper about the CASK mutation published by Konark Mukherjee, assistant professor at the Fralin Biomedical Research Institute and one of the few researchers in the world to study how the CASK mutation impacts the brain.
Mukherjee referred the family to the Fralin Biomedical Research Institute Neuromotor Research Clinic, directed by associate professor Stephanie DeLuca. For nearly a decade, the clinic’s research team has developed intensive therapies for children with disorders that alter brain development or function. DeLuca’s research team also has a long history of working with children with varied types of neuromotor impairments, including rare diseases.
Evie received one month of an intensive therapy program aimed at helping her develop the ability to sit upright and coordinate movements. In spring of 2020, Evie could not sit up on her own. Today, she can stand with assistance and is working to use a walker. She has worked hard to exceed expectations, her family said.
Similar to Evie, 3-year-old Sarah Hattersly crossed the Atlantic to reach Roanoke, Virginia, in 2021.
After four weeks of intensive therapy at the Neuromotor Research Clinic, Sarah was able to understand simple sentences, smile, play with toys, and use sign language to say “more” and “all done” – all things she previously could not do.
“We believe intensive therapies help children learn because threshold levels of input are reached to allow neural connections in the brain to be made,” DeLuca said. “We know a lot about how to help children improve, but to get the complete picture to explain why it works, we truly need to understand the neural mechanisms through future research.”
DeLuca and her colleagues have published studies evaluating intensive therapies for CASK-related disorders and other rare diseases, including Kernicterus, a neurological disorder caused by excess bilirubin in the blood during infancy that impacts brain development.

This month, researchers published a new study showing that CASK gene mutations do not impact normal brain development in utero, but rather lead to an early degeneration of otherwise healthy neurons. This discovery could help researchers edge closer to finding a therapeutic intervention, according to Mukherjee.
“This completely changes the way we think about intervening in CASK disorders,” Mukherjee said. “With the understanding that the symptoms result from loss of neurons, it becomes crucial to figure out the toxic biochemical process that is killing the neurons and to stop it.”
Investigating rare genetic diseases
More than 70% of reported rare disease are caused by mutated or missing genes. Studying rare genetic disorders helps researchers understand the affected gene's purpose, while also casting a framework for developing new therapies.
DiGeorge syndrome
Anthony-Samuel LaMantia, a world-renowned geneticist, professor, and director of the research institute’s Center for Neurobiology Research, has for decades focused on DiGeorge syndrome, a disorder occurring when a small part of chromosome 22 is missing. This deletion – which affects an estimated one in 4,000 Americans – causes the brain, heart, head, and limbs to improperly develop.
This month, LaMantia and his research team published a new study revealing how DiGeorge syndrome hinders early cellular interactions underlying the development of pain-sensing and movement-sensing neurons in the cranial nerve. In 2020, the lab published findings exploring the role of motor neurons in coordinating eating and swallowing behaviors.
LaMantia and his laboratory have also analyzed how the chromosome 22 deletion associated with the disorder disrupts cerebral cortex development. Children with DiGeorge syndrome are at higher risk for being diagnosed with autism spectrum disorder when they are young, or schizophrenia in adolescence and early adulthood. LaMantia, Tom Maynard, research associate professor, and Daniel Meechan, research assistant professor, investigated this connection in mouse genetic models. They discovered that the chromosomal deletion underlying DiGeorge syndrome selectively diminishes connections between key areas of the cerebral cortex that mediate cognitive behaviors. A follow-up study showed that mitochondrial dysfunction may play a role in disrupting these important cortical connections.
“Our goal is to learn about the causes of these DiGeorge syndrome symptoms to help children as early in life as possible,” LaMantia said.
Brugada syndrome
Researchers in the Fralin Biomedical Research Institute's Center for Vascular and Heart Research are also studying rare diseases that negatively impact electrical signaling in the heart.
It’s estimated that one in 2,000 Americans are living with Brugada syndrome – a rare disease accounting for 20% of sudden cardiac death cases. Patients with Brugada syndrome usually have mutations in the SCN5A gene, which encodes proteins that regulate sodium channel function in the heart.
Researchers led by Steven Poelzing, associate professor at the Fralin Biomedical Research Institute and co-director of the Virginia Tech Translational Biology, Medicine, and Health Graduate Program, are studying Brugada syndrome to understand how faulty sodium channels influence cardiac function and heart rhythms.
Long QT syndrome
Poelzing’s laboratory is also investigating a similar disease, Long QT syndrome, which can be caused by environmental factors or mutations in the same SCN5A gene as Brugada cases. Patients with Long QT have a lengthened Q-T interval, a measurement of how long it takes for the heart’s electrical pathways to repolarize after a heartbeat.
“Many people living with Brugada or Long QT are asymptomatic, which can make it harder to detect and prevent dangerous arrhythmias,” Poelzing said.
Poelzing’s research into electrical pathways in the heart has provided some insight into the precursors for sudden cardiac death and dangerous arrhythmias associated with these conditions.
They recently discovered that in a model of Long QT syndrome, elevated blood sodium levels, heart tissue swelling, and sodium channel dysfunction needed to occur simultaneously to produce dangerous arrhythmias.
“We’ve found a potentially cost-effective strategy to mitigate sodium channel-related diseases by managing blood sodium levels through diet and hydration, and preventing cardiac edema,” Poelzing said.
Studying rare, aggressive forms of cancer
Nearly one in eight adult cancer patients in the U.S. have a rare form of the disease. Rare cancers can be challenging to identify, often resulting in delayed diagnosis after symptom onset. Even after diagnosis, treatment options and clinical trials are usually more limited for patients with rare cancers.
Glioblastoma
Glioblastoma is a fast-growing, aggressive malignant brain tumor that can cause headaches, personality changes, nausea, stroke-like symptoms, and death. Fewer than one in 10 patients diagnosed with this rare cancer survive five years after diagnosis.
A research team led by Zhi Sheng recently described a promising new approach to overcome chemoresistance in this lethal brain cancer. The researchers developed a three-pronged treatment approach involving two peptides combined with a front-line chemotherapy, temozolomide. Sheng is an assistant professor at the Fralin Biomedical Research Institute and a Virginia Tech Cancer Research Alliance member.
Samy Lamouille, an assistant professor at the Fralin Biomedical Research Institute, co-founded Acomhal Research Inc. with Rob Gourdie, professor and director of the Center for Vascular and Heart Research, to advance treatments for rare cancers, including glioblastoma, by targeting cancer stem cells.
“Cancer stem cells within tumors display high resistance to cancer therapies. If these cells are left behind after treatment, they contribute to cancer relapse by seeding the growth of new tumors,” Lamouille said.
The company is developing a peptide drug that targets cancer stem cells in glioblastoma, with the goal of combining this novel therapy with conventional treatments to eradicate more cancer cell types and prevent tumor recurrence.
Triple negative breast cancer
For 40% of patients diagnosed with triple negative breast cancer, the cancer will come back and spread – even after treatment. Patients with this form of cancer are more likely to develop tumors in other organs, such as the brain or lungs.
Metastasis in this form of cancer is caused by cancer cell plasticity that enables the cells to acquire more invasive traits, Lamouille explained. In 2020, Acomhal secured funding to study if a new molecule can prevent triple negative breast cancer cells from escaping primary tumors. This drug targets proteins that cancer cells use to degrade macromolecules in the tumor microenvironment. This degradation allows invasive cancer cells to break away from the tumor and colonize new tissues.
“By targeting these proteins involved in cancer cell invasion, our goal is to limit the ability of cancer cells to metastasize, halting this devastating disease," Lamouille said.
Diffuse intrinsic pontine glioma
Nearly 100% of children diagnosed with diffuse intrinsic pontine glioma (DIPG), an aggressive and rare form of pediatric brain cancer, die within five years of diagnosis. The treatment-resistant tumors are often inoperable because of their location in the brainstem.
An interdisciplinary research team led by Jennifer Munson, associate professor at the Fralin Biomedical Research Institute, are evaluating the combined use of focused ultrasound – hypothesized to improve drug delivery across the blood brain barrier – with sonodynamic therapy and chemotherapy to treat DIPG. The research team received a 2022 Fralin Biomedical Research Institute Seale Innovation Fund pilot grant to initiate the study.
Last year, the Fralin Biomedical Research Institute recruited assistant professor Jia-Ray Yu to launch a new laboratory on the Children’s National Research & Innovation Campus investigating DIPG and other cancers.
According to Yu’s research, 80% of these rare gliomas begin with a single cell that has a histone gene defect, spurring a domino-like reaction that triggers healthy brain cells to become cancerous ones. Yu’s new laboratory combines multiple research techniques to develop and evaluate new pharmaceutical alternatives to chemotherapy and radiation.
Pediatric stroke patients with hemiparesis gain neuromotor skills through intensive therapy
Perinatal stroke affects roughly one in 2,500 babies born in the United States every year. It is also the leading cause of hemiparesis – a condition that causes children to have asymmetrical abilities on the two sides of their body.
Since it was founded in 2013, the Neuromotor Research Clinic has helped many infants and children recovering from stroke gain motor and cognitive skills through intensive bursts of constraint-induced movement therapy.
The researchers apply a lightweight cast to the child’s non-impaired arm, compelling them to rehabilitate the side of their body impacted by the stroke. The casting occurs jointly with a four-week intensive occupational therapy protocol.

Four years ago, the first Phase III multicenter pediatric stroke recovery trial began. Led by Sharon Landesman Ramey, professor and distinguished research scholar at the Fralin Biomedical Research Institute, and Warren Lo at Nationwide Children’s Hospital, the trial is evaluating the therapy in 240 children nationwide. The institute’s Neuromotor Research Clinic serves as the treatment implementation core for the 14-site trial.
Ramey and DeLuca recently demonstrated that higher doses of constraint-induced movement therapy – 20 three-hour sessions over four weeks – yielded significant and lasting improvements in everyday function of the patients’ arms and hands. The findings were reported in Pediatrics in 2021.
Studying rare diseases at the Fralin Biomedical Research Institute
Researchers across the world continue to make progress in identifying new ways to diagnose, treat, and even prevent a wide range of rare diseases – yet 95% of the conditions still lack effective treatments.
“Although the incidence of any individual rare disease by definition is low, as defined in the Orphan Drug Act as affecting fewer than 200,000 people, the fundamental scientific discoveries that emerge through their study provides immense value," said Michael Friedlander, executive director of the Fralin Biomedical Research Institute and Virginia Tech’s vice president for Health Sciences and Technology.
“In addition to affecting more than 30 million Americans who need help from discoveries to advance treatments and cures, rare diseases offer an important biological window into cellular and molecular processes that manifest in other disorders that have much higher prevalence. It’s vital that we continue to investigate the mechanisms and treatments of rare diseases to advance our understanding of human lifespan development, and ultimately to help patients with rare diseases while also providing insights and informing new therapeutics for treating more common disorders alike.”




