Neuroscientists explore differences in mitochondria of memory cells in the brain with new NIH funding
How do we recall faces, names, and where and when we met someone? Shannon Farris with the Fralin Biomedical Research Institute at VTC is studying the bioenergetics, molecular cues, and distinct cell types that encode social memories in the brain’s hippocampus.

Have you ever seen someone you recognized, but couldn’t recall their name or how you knew them?
As you strain to recollect the details, a pea-sized clump of neurons nestled in your hippocampus is working hard to connect the dots. This brain region, coined CA2, uniquely encodes social memories in mammals. Without it, mice can remember familiar inanimate objects – but not friends or foes they’d met before.
Now, with a five-year, $2-million National Institutes of Health grant, Shannon Farris, assistant professor at the Fralin Biomedical Research Institute at VTC, is mapping out the diverse bioenergetic and molecular characteristics of CA2 neuronal circuits to learn more about how social memories are formed, stored, and forgotten.
“Impaired social memory is a phenotype of numerous neurological disorders, ranging from autism spectrum disorder to schizophrenia and Alzheimer’s disease,” Farris said. “By unraveling the molecular nuances underlying healthy memory storage, we aim to pinpoint a host of potential interventional targets for neurodevelopmental, neurocognitive and neurodegenerative disease.”
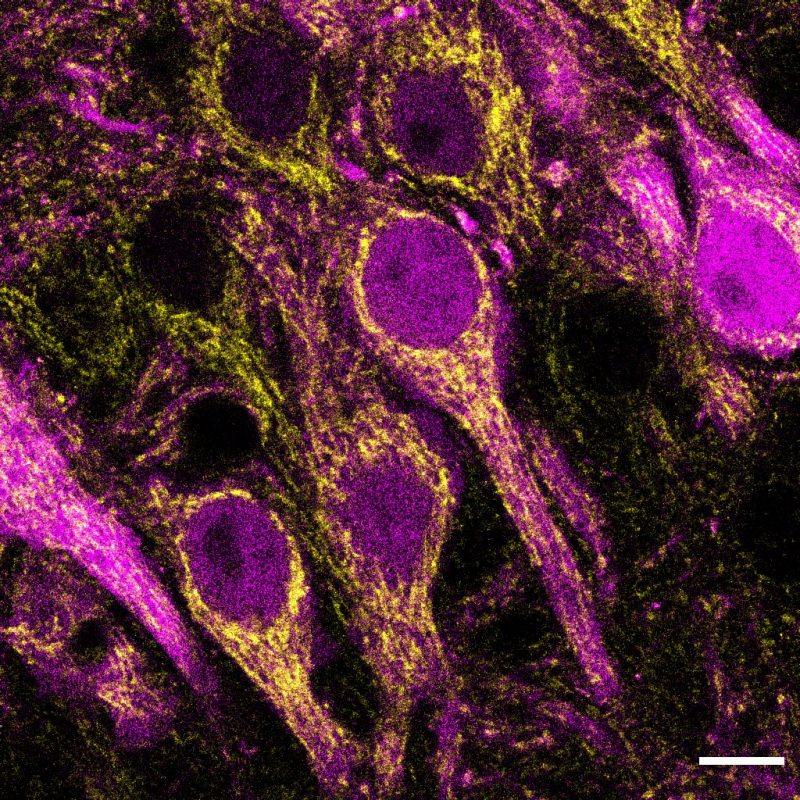
The neurons in this brain region are energetically demanding – even more so than neighboring cells within the hippocampus. As a result, their mitochondria are bigger and more abundant, Farris explained.
But when her lab took a closer look at the bioenergetics of individual neurons in CA2 in mice, they made an unexpected discovery.
“I’d never seen anything like it before – and I’ve spent years examining this specific brain region,” Farris said.
Within a single CA2 neuron, there were different types of mitochondria based on the organelle’s location, with distal dendrites harboring molecularly and structurally distinct mitochondria compared with more proximal dendrites or neighboring neurons.
“We know that different organs, tissues, and brain regions have unique mitochondria. But here we uncovered mitochondrial heterogeneity within a single brain cell,” said Farris, who also has an appointment in the Virginia-Maryland College of Veterinary Medicine.
She hypothesizes that these unusual mitochondrial characteristics may be influencing this brain region’s plasticity – or ability to rapidly modify synapses, neurochemical portals that mediate communication between neurons.
Katy Pannoni, a postdoctoral associate in Farris’s lab, presented the findings this week at the Society for Neuroscience conference.
Over the next five years, Farris and her team will combine a variety of new techniques and technologies – including expansion and scanning block face electron microscopy to develop 3D neuronal reconstructions, and real-time metabolic analysis – to describe the bioenergetic and molecular properties of CA2 neurons. The researchers will also genetically knock out specific mitochondrial genes to better define how certain mitochondrial properties uniquely impact social memory and behavior in mice.
The Farris lab is currently recruiting a research assistant to join the lab to assist with this research.




