Researchers use cell imaging and mathematical modeling to understand cancer progression

Cell division is a fundamental process that organisms need to reproduce, grow, and make repairs. But when an error disrupts this complex biological process, cellular abnormalities can lead to diseases, such as cancer, where cells are enabled to grow and divide out of control.
Using a combination of experiments and mathematical modeling, a team of researchers from the Virginia Tech Department of Biological Sciences in the College of Science and the Fralin Life Sciences Institute are beginning to unravel the mechanisms that lie behind tetraploidy - a chromosomal abnormality that is often found in malignant tumors.
Their findings were published on April 29 in eLife, an open-access journal that is dedicated to life science research.
“Our study used fixed cell analysis, live cell imaging, and mathematical modeling to help us better understand the role of tetraploidy in tumor formation and progression. This work lays the foundation for future studies to really understand the link between tetraploidy and cancer. If we know what is happening in tumors, then we can have a better idea of how to develop better treatments for them,” said Nicolaas Baudoin, the lead author on the study and a recent Ph.D. graduate in the Department of Biological Sciences and the BIOTRANS program, an interdisciplinary graduate program of biologists and engineers.
Every human ‘parent’ cell holds two copies of each chromosome. Before cell division begins, every chromosome is duplicated so that the genetic information can be equally distributed between two ‘daughter’ cells. But if the parent cell fails to complete cell division, all four chromosomes are allocated into one daughter cell, thus making the cell tetraploid.
When tetraploid cells acquire twice the number of chromosomes, they also acquire twice the number of centrosomes. Among their organizational and structural roles, centrosomes are key to forming microtubules and spindle fibers, which work to pull chromosomes apart during cell division. With the overabundance of centrosomes, the chromosomes are pulled in many different directions and cell division can have abnormal results.
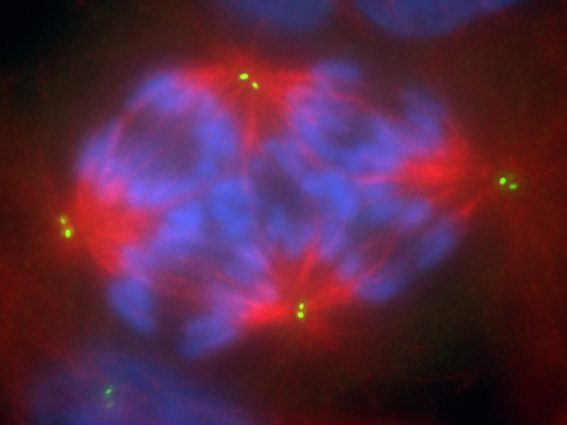
Previous studies had suggested that these extra centrosomes may cause tumor formation, induced by tetraploidy. But then, the Virginia Tech team came across two studies in cancer progression models, which showed that the cells gained extra centrosomes initially, but ended up losing them over time.
“The main goal of our study was to verify that tetraploid cells lose the extra centrosomes, examine the dynamics of this process, and uncover the mechanism that causes this centrosome loss from tetraploid cells,” said Daniela Cimini, a professor from the Department of Biological Sciences and the co-director of BIOTRANS.
Using live cell imaging and fixed cell analysis in an in vitro model, the team confirmed that tetraploid cells did lose the extra centrosomes that they had gained during tetraploidization.
In experiments guided by mathematical modeling, they concluded that centrosome loss happens when dividing tetraploid cells cluster their extra centrosomes asymmetrically. As a result, one of the daughter cells will inherit one centrosome - instead of two - which will allow the cell to suffer fewer cell division failures and produce more cells in the long term.
This finding can explain how certain cancers may first gain extra centrosomes during tetraploidization, but then lose them at later stages. This indicates that the causal relationship between tetraploidy and cancer needs further investigation.
The mathematical model also found that the only cells that could sustain long-term survival with extra centrosomes were cells that could successfully and consistently cluster these centrosomes in two groups during cell division. These predictions were tested experimentally and revealed a mechanism that explains why certain cancer cells survive despite their additional centrosome count. And if cells failed to cluster their extra centrosomes effectively, the next generation of daughter cells died.
Baudoin and Cimini agree that this level of mechanistic understanding was only possible thanks to their collaboration with Jing Chen, a mathematical biologist and assistant professor of biological sciences in the Virginia Tech College of Science.
“Built upon experimental measurements, the mathematical model paints a continuous and detailed picture about how the cells’ centrosome numbers change. This allows us to see information that cannot be measured by experiments.” said Chen, an affiliated faculty member of the Fralin Life Sciences Institute and BIOTRANS.
Next, the team would like to take advantage of their model to better understand the cellular dynamics within three-dimensional cultures and real tumors.
In their in vitro system, the team could get a sense of what was happening within the cells by tracking and imaging them, but this cannot be done in more complex systems like real tumors. With their newest model and previous data, the team will be able to make some compelling predictions.
According to Chen, the success of present and future cancer studies could be attributed to a unique, but all important, collaboration between researchers in the fields of biology and mathematics.
“This hand-in-hand collaboration between experimentalist and modeler is very important - and it’s a great approach for modeling biological studies. The process requires a lot of close communication between us. When that’s done correctly, it can be very powerful,” said Chen.
- Written by Kendall Daniels




