Fralin Biomedical researchers shed light on how to protect optic nerve from genetic harm
Konark Mukherjee, an assistant professor with Fralin Biomedical Research Institute, and colleagues have revealed new insight into the most common cause of childhood blindness.

Virginia Tech scientists at the Fralin Biomedical Research Institute at VTC who study a gene called CASK have developed a new insight about the most common form of inherited childhood blindness known as optic nerve hypoplasia.
The disease occurs when the optic nerve — which transmits visual information from the retina of the eye to the brain — is underdeveloped. It is the most common cause of childhood blindness in developed nations and there are few treatment guidelines.
Using a mouse model of the disease developed by researchers at the Fralin Biomedical Research Institute, researchers say a “noncell autonomous” effect — where the disease occurs because of factors or conditions arising in the cellular environment — may be at work.
The study, which was first published in the journal Investigative Ophthalmology and Visual Science, suggests beyond targeting the damaged retinal cells and their axonal output lines to the brain – the optic nerve, researchers may want to focus on treatment strategies that address an unhealthful molecular environment in the neighborhood of those cells and their processes.
Even better, researchers found the optic nerve in mice with the CASK defect is normal at birth, which suggests that there may be a therapeutic window to help children born with the disease before the damage has manifested.
“The first thing we wanted to know is not which cells are dying, but which cells were causing the problem,” said Konark Mukherjee, an assistant professor with Fralin Biomedical Research Institute whose lab may be the only one in the country focused on studies of the CASK gene.
“It is important to understand where the damaging effect is coming from in optic nerve hypoplasia, which is challenging because the mechanism that underlies its onset and progression remains unknown.”
The brain devotes more space to vision than to all other senses combined, according to the Dana Foundation, which is coordinating a global education initiative to focus attention on treatments, preventions, and possible cures for brain diseases.
To find out more about childhood blindness and optic nerve hypoplasia, Mukherjee and Michael A. Fox, a professor at the Fralin Biomedical Research Institute and director of the institute’s Center for Neurobiology Research, utilized a mouse model to understand the effects of CASK deficiency in retinal cells and optic nerve development.
In previous studies, CASK has been linked with microcephaly, intellectual disabilities, and visual impairments in people. Now, the scientists discovered that reduction in CASK in mice adversely affects retinal ganglion cells, but even the complete absence of CASK did not kill the cells.
“We suspect that CASK is important for optic nerve development, but if it were critical for cell survival, then the cells should have died without it,” Mukherjee said. “That did not happen. This tells us those surviving cells are receiving the effect and exposure to CASK from somewhere else and some other cell type.”
Because of this noncell autonomous effect — where the cellular problems occur because of factors or conditions arising from neighboring cells — creative opportunities for effective interventions are possible, the researchers said.
“In addition to neurons, CASK is present in other brain cell populations, such as glial and endothelial cells,” said Fox, who is also a professor in the Department of Biological Sciences at Virginia Tech. “Abnormal functioning in these cell populations is likely to affect the health and development of the optic nerve.”
Additional study of CASK mutant mice will be useful for understanding optic nerve hypoplasia in children born with the disease and finding new ways to help.
“It is similar to a heart patient with atherosclerosis because of high cholesterol – the disease is triggered by conditions in the liver and diet, not necessarily the heart,” Mukherjee said. “It is likely the same relationship for optic nerve hypoplasia — that means we can do something about it. It is also exciting that we found the optic nerve appears normal at birth in the mouse deteriorating postnatally. That means there may be a time window to protect or repair the optic nerve before it is lost.”
The research was supported by grants from the National Eye Institute at the National Institutes of Health.
Virginia Tech translational biology, medicine, and health graduate student Paras Patel; former doctoral student and research assistant Alicia Kerr, now working at the National Institutes of Health; and Chen Liang, a doctoral student in the Department of Biological Sciences at Virginia Tech, participated in the research, as did Leslie LaConte, an associate professor at the Fralin Biomedical Research Institute and assistant dean for research at the Virginia Tech Carilion School of Medicine. In addition, Ching-Kang Chene and Veeral Shahof of the Baylor College of Medicine contributed to the study.
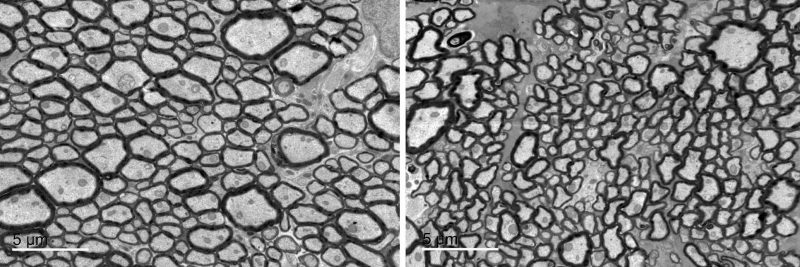
Electron micrograph of cross-section of optic nerves; left panel (normal) and right panel (Cask mutant). Electron micrographs display cross-sections of optic nerves as dark circles. Normal optic nerves are at left while the right panel shows thinner axons — the threadlike part of the optic nerve — in mice with CASK mutations. CASK mutations in human are known to cause optic nerve hypoplasia.