Zebrafish study reveals developmental mechanisms of eye movement
Albert Pan (left), an associate professor at the Fralin Biomedical Research Institute at VTC, and Manxiu "Michelle" Ma, a research associate in the Pan lab, were part of a team of scientists who worked with zebrafish to discover that genes linked to autism spectrum disorder and other brain abnormalities may be playing a role in people who cannot control their eye movements.

Researchers studying zebrafish have found that genes linked to autism spectrum disorder and other developmental brain abnormalities may be playing a role in people who cannot control their eye movements.
The findings, to publish this week in The Journal of Neuroscience — the official journal of the nearly 40,000-member Society for Neuroscience — underscore the importance of the Down Syndrome Cell Adhesion Molecule-Like 1 gene in the development of eye movements.
The study utilizes zebrafish as a model to investigate neural circuits underlying human eye movement disorders. In recent years, zebrafish have emerged as an important tool for biomedical research, providing insight into the pathogenic mechanisms of complex behavioral disorders.
“We can put zebrafish under a microscope, especially in the larval stage, and observe cells moving, eyes developing, and all kinds of exciting neuronal activity,” said Albert Pan, an associate professor at the Fralin Biomedical Research Institute at VTC, who led a multi-university team of investigators in the study. “Because people and fish share similarities in brain structure and genes, this simple organism provides us with an extraordinary window to understand the common neural pathway and brain activity patterns underlying eye movements.”
As vertebrates with many similarities in development and body plan, fish and people share many genes and proteins, including the Down Syndrome Cell Adhesion Molecule-Like 1 gene, called dscaml1 in zebrafish. In people, loss of this gene is one of many mutations that have been linked to autism spectrum disorder and malformations in the cortex of the brain. However, how dscaml1 deficiency may contribute to behavioral deficits had been mostly unknown.
In the study, lead authors Manxiu "Michelle" Ma, a research associate in the Pan lab; Alexandro Ramirez, a postdoctoral fellow in the Emre Aksay lab at Weill Cornell College of Medicine; and Tong Wang, a former postdoctoral fellow in the Pan lab, analyzed how dscaml1 deficiency affects neural development and eye movements in zebrafish.
By video-tracking eye movements in the larval zebrafish, the scientists found that dscaml1 mutants have a host of eye movement deficits, including rapid fatigue, impaired visual fixation, and inability to perform a type of fast, ballistic eye movement called saccade.
Saccades are important for quickly shifting our gaze to different points of interest. Patients with developmental deficits in saccade generation (ocular motor apraxia) often have difficulty reading or performing other tasks that require frequent gaze shifting. The researchers further used a powerful imaging technique called two-photon calcium imaging to visualize neuronal activity in the living animal's brain during saccades and were able to identify impaired neural pathways that correspond to the behavioral deficits.
The researchers said that many characteristics of the zebrafish dscaml1 mutants are similar to human ocular motor apraxia, and further research in the zebrafish model may lead to discoveries into complex diseases.
"Many behavioral problems are characterized by abnormal eye movements and by how people visually perceive faces and emotions," said Pan, who is also the Commonwealth Center for Innovative Technology Eminent Research Scholar in Developmental Neuroscience and an associate professor in the Department of Biomedical Sciences and Pathobiology and the Department of Psychiatry and Behavioral Medicine at Virginia Tech. "With a simple organism like zebrafish and a relatively simple neural circuit that generates and controls eye movements, we can figure out how genes affect development, and hopefully that will help us understand how genes affect psychiatric disorders."
In addition to scientists from Virginia Tech and Weill Cornell College of Medicine, faculty from Harvard University, Massachusetts General Hospital, Augusta University, and New York University Langone School of Medicine participated in the research.
The work was supported by the the work was supported by the National Eye Institute and the National Institute of Neurological Disorders and Stroke, both parts of the National Institutes of Health, the Simons Global Brain Initiative, the Commonwealth of Virginia Center for Innovative Technology, Virginia Tech, the Fralin Biomedical Research Institute, and Augusta University.
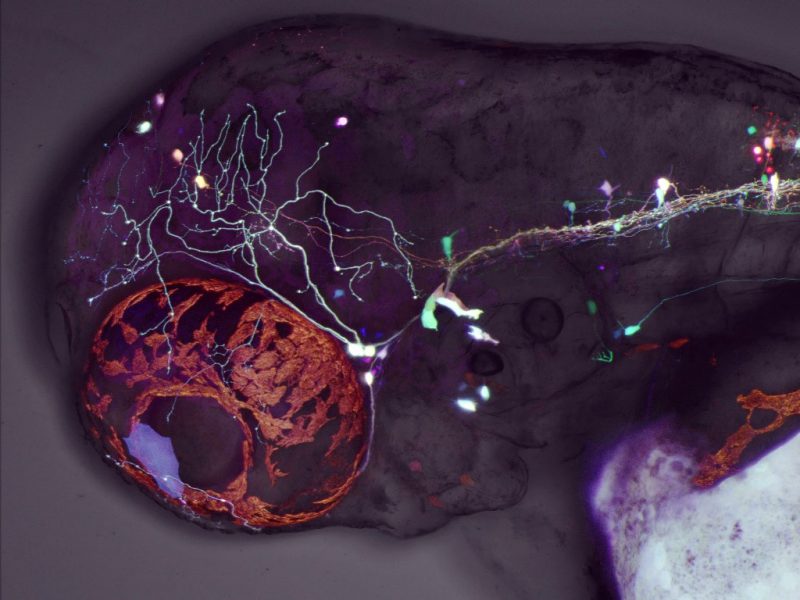
This is a fluorescent image of a live larval zebrafish, viewed from the side. Fluorescent neurons are seen next to the eye (orange oval) and along the body.