Virginia Tech Carilion Research Institute teams converge on strategies to defeat McCain’s form of brain cancer
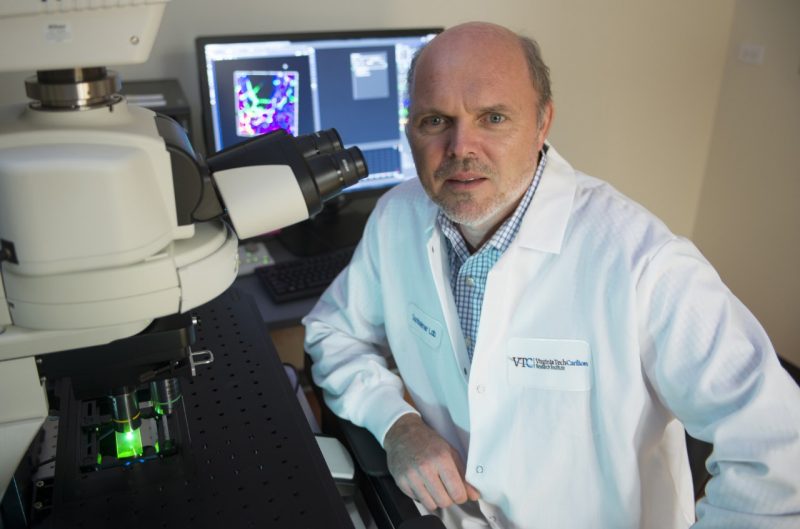
Rob Gourdie, Samy Lamouille, Harald Sontheimer, and Zhi Sheng [from left] have established research programs into the type of cancer recently diagnosed in U.S. Sen. John McCain.
![Glioblastoma researchers Rob Gourdie, Samy Lamouille, Harald Sontheimer, and Zhi Sheng [from left] have established research programs into the type of cancer recently diagnosed in U.S. Sen John McCain.](/content/news_vt_edu/en/articles/2017/07/glioblastoma-vtcri-0724/_jcr_content/article-image.transform/m-medium/image.jpg)
Research teams at the Virginia Tech Carilion Research Institute from three colleges — Engineering, Science, and Veterinary Medicine — are developing new approaches to treat glioblastoma, the aggressive form of brain cancer recently diagnosed in U.S. Sen. John McCain.
About half of glioblastoma patients die within the first 12 to 18 months of diagnosis, according to the National Institute of Neurological Disorders and Stroke.
“Overall, Virginia Tech Carilion Research Institute is positioned to have important impact in developing innovative therapies for treating glioblastoma,” said Michael J. Friedlander, founding executive director of the research institute and vice president for health sciences and technology at Virginia Tech. “With complementary research approaches in several laboratories here, we are committed to bringing cutting-edge science to bear on this devastating disorder that has evaded substantial progress for too long.”
The institute has invested resources and recruited top talent to focus on the challenge of brain cancer, Friedlander said.
“Although the prevalence of glioblastoma is not as great as several other cancers, the lack of good therapeutic options is a major challenge and one in which the research institute with its strengths in brain research, cell and structural biology, and focus on translational science can make a difference — and we plan to do so,” Friedlander said.
Researchers at the institute attacking the problem include Rob Gourdie, Harald Sontheimer, Zhi Sheng, and Samy Lamouille.
Gourdie is the Commonwealth Eminent Scholar of Regenerative Medicine and professor of biomedical engineering and mechanics in the College of Engineering; Sontheimer is the I.D. Wilson Chair and professor of neuroscience, and executive director of the School of Neuroscience in the College of Science; Sheng is an assistant professor of biomedical science and pathobiology in the Virginia-Maryland College of Veterinary Medicine; and Lamouille is a research assistant professor at the VTCRI.
Rob Gourdie [left] and Samy Lamouille have started a company in an attempt to speed medical discoveries to patients. The company, Acomhal Research, is based in the Regional Acceleration and Mentoring Program Building in Roanoke.

Gourdie, who directs the VTCRI Center for Heart and Regenerative Medicine Research, and Lamouille have taken an approach to target cancer stem cells that form the seeds of new tumors in glioblastoma, using a novel compound called a JM peptide that targets the molecules that form the bridges between neighboring glioblastoma cells.
The work already has led to a new company, Acomhal Research, based in the new Regional Acceleration and Mentoring Program (RAMP) building in Roanoke, Virginia.
In 2015, the research institute established the Center for Glial Biology in Health, Disease, and Cancer to tackle many of the brain disorders where glial cells play a central role, including the urgent challenge to develop new, effective therapies for glioblastoma.
Sontheimer, the director of the center, and his team are taking an innovative approach to personalized cancer medicine by evaluating the effectiveness of an FDA-approved drug sulfasalazine to target a molecular transport process that is particularly highly expressed in some patients' glioblastomas to prevent further brain damage due to the tumor destroying neighboring brain tissue through a process called excitotoxicity.
Harald Sontheimer, the director of the Virginia Tech Carilion Research Institute Center for Glial Biology in Health, Disease, and Cancer, has worked on a novel therapy that uses an existing FDA-approved drug to slow tumor growth.
The drug is routinely used for another purpose — to treat inflammatory bowel disease.
In preclinical studies, tumor-bearing mice treated with sulfasalazine twice daily showed a reduction in seizures and slowed tumor growth.
Moreover, these tumors often cause seizures and kill surrounding brain tissue through the release of toxic glutamate. In a clinical pilot trial with nine patients who received acute doses of sulfasalazine while being imaged for glutamate, results showed sulfasalazine was able to inhibit toxic glutamate release from the tumor.
As a result, together with the Wake Forest Baptist Health’s Comprehensive Cancer Center in North Carolina, researchers have begun a clinical trial that will treat patients with sulfasalazine in conjunction with the chemotherapeutic temozolomide and radiation in hopes to slow tumor growth.
Zhi Sheng, an assistant professor at the Virginia Tech Carilion Research Institute [right], with Pratik Kanabur, a medical student researcher with the Virginia Tech Carilion School of Medicine, who studied glioblastoma under Sheng’s supervision at the Research Institute.
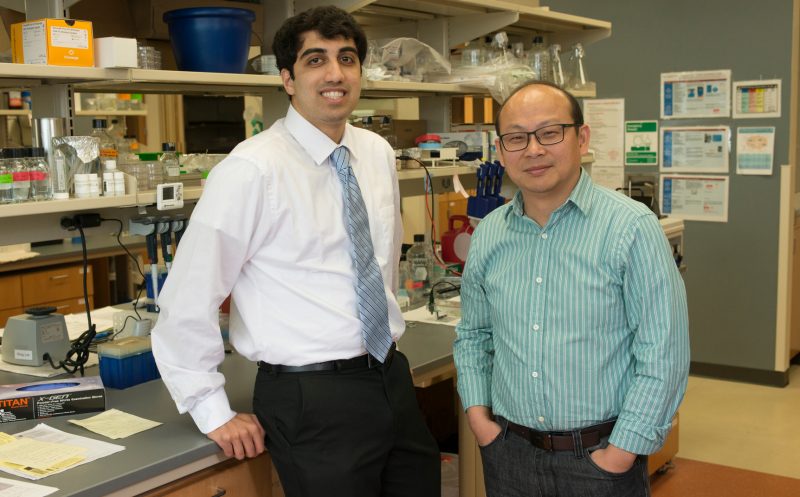
Sheng, Gourdie and Lamouille, using glioblastoma stem cells isolated from freshly obtained human tumors in collaboration with neurosurgeon Gary Simonds’ team at Carilion Clinic, discovered stem cells isolated from patient tissues could potentially be used to target glioblastoma.
They focused on the molecular substrates of the cellular junctions between glioblastoma cells to determine if this might re-sensitize the cancerous cells to treatment with temozolomide. This work has led to a clinical trial to treat glioblastoma in dogs in collaboration with John Rossmeisl, a professor of neurology and neurosurgery in the Department of Small Animal Clinical Sciences in the Virginia-Maryland College of Veterinary Medicine.
“Our discovery shows different patients have different types of cancer stem cells, and those cancer stem cells respond to therapies differently,” Sheng said. “It would be great for these patients if we could start by moving the clock, even just a little bit, and give them even a little more time.”
In a study in the journal Oncotarget, featured in the video below, the Sheng team analyzed levels of messenger RNA from stem cells isolated from individual glioblastoma tumor specimens obtained through the division of neurosurgery in the Department of Surgery at Carilion Clinic.
“There are cases where a healthy 50-year-old wakes up with a headache and it turns out to be from a brain tumor,” said Pratik Kanabur, a medical student researcher with the Virginia Tech Carilion School of Medicine who conducted research under Sheng’s supervision at the VTCRI. “Glioblastoma can strike any healthy person and limit their life expectancy to about a year, even with aggressive treatment. Patients desperately need better options, and we think one approach is to go to the source of the cancer, the glioblastoma stem cells.”
The researchers also used data from more than 500 glioblastoma patients contained in The Cancer Genome Atlas, a catalog of gene mutations responsible for cancer maintained by the National Cancer Institute and the National Human Genome Institute.
Collaborating with Sheng and Gourdie on the study were Kanabur, research associate Sujuan Guo, Simonds, the chief of neurosurgery at Carilion Clinic; Deborah Kelly, an associate professor at VTCRI; and Scott Verbridge, an assistant professor with the Virginia Tech-Wake Forest University School of Biomedical Engineering and Sciences.







